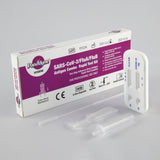
COVID-19 & Influenza A+B Antigen Combo Rapid Test Kit
SKU: PDCA2T-U
Overview
Both COVID-19 and influenza A/B viruses (Flu A/B) can cause acute respiratory infections, and people are generally susceptible. These three viruses are highly contagious, spread quickly, have a short incubation period, and have a high incidence.
With this combination test, healthcare workers can provide an accurate diagnosis to patients and treat them with the appropriate measures.
COVID-19 & Influenza A+B Antigen Combo Rapid Test Kit Benefits
- Results ready in 15 minutes
- Accurate diagnostic tool for active infection
- Easy to administer and read results
- Affordable, no need for instrument, highly portable
- Enable testing on a massive scale
- For healthcare workers use only
Kit contents
- Test cassette
- Sterile swab
- Antigen extraction buffer
- Extraction tube
Organisations and businesses who already are or wish to implement a test tracking program, please click on the link below to download the Panodyne COVID-19 Antigen and Influenza test declaration form for subjects to sign and for your records. The declaration form can be used for research and consent form purposes only.
Intended Use
COVID-19 & FLU A/B Antigen Test Kit (Colloidal Gold) is used for in vitro qualitative detection of 2019 Novel Coronavirus antigen and influenza A/B antigen in human nasopharyngeal swab samples.
Product Information
- Product: SARS-CoV-2/FluA/FluB Antigen Test Kit (Colloidal Gold)
- Pack formats: 1 Test/Box 24 Tests/Box
- Test Type: Antigen Test
- Test Principle: Colloidal Gold Method
- Sample Type: Nasopharyngeal Swab
- Sample Volume: 3 Drops of Extracted Solution (about 100μl) Each Well
- Qualitative / Quantitative: Qualitative
- Test Time: 15 Mins
- Operation Temperature: Room Temperature (15-30°C)
- Storage Temperature: Room Temperature or Refrigerated (2-30°C)
- Shelf Life (Unopened): 24 Month
Storage and stability
- The test kit can be stored at room temperature or refrigerated (2-30°C). The test device is stable through the expiration date printed on the sealed pouch. The test device must remain in the sealed pouch until use.
- Do not freeze.
- Do not use beyond the expiration date.
- After opening the sealed pouch, use the test as soon as possible within 60 minutes.
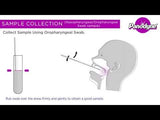
Specimen Collection
- SARS-CoV-2/FluA/FluB Antigen Test kit (Colloidal Gold) can be performed using nasopharyngeal swab.
- Testing should be performed immediately after specimen collection.
- Bring specimens to room temperature prior to testing.
- If specimens are to be shipped, they should be packed in compliance with local regulations covering the transportation of etiologic agents.
Test procedure
- Insert a swab through the nasal cavity to the nasopharynx of the patient, reaching the surface of the posterior nasopharynx when resistance is encountered.
- Gently rub and roll the swab for several seconds to absorb secretions.
- Slowly remove nasal swab while rotating it.
- Put the swab specimen into the antigen extraction tube pre-added with the antigen extraction buffer.
- Rotate the swab about 10 times while pressing the swab head against the tube wall to release the antigen in the swab.
- Let it stand for about 1 minute.
- Remove the swab while squeezing the tip of the swab so that as much liquid in the swab can be discharged as possible. Dispose of used swabs in accordance with biohazard waste disposal methods.
- Install the dripper on the antigen extraction tube and cap it tightly, and let it stand for about 1 minute.
- Remove the test cassette from the sealed foil pouch and use it as soon as possible. Place the test device on a clean and level surface. Transfer 3 drops (about 100μl) of the mixed liquid to each sample well of the test card (or use a pipette to add 100μl), and start the timer.
- Wait for the test result of the reagent. The result should be read in 15 minutes. Do not interpret the result after 20 minutes.
Interpretation of result: COVID-19 Antigen test kit
Negative:
If only the C band is present, the absence of any burgundy colour in the T band indicates that no COVID-19 antigen is detected in the specimen. The result is negative.
Positive:
In addition to the presence of C band, if T band is developed, the test indicates for the presence of COVID-19 antigen in the specimen. The result is COVID-19 positive.
Invalid:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Interpretation of result: Flu A/B Antigen test kit:
Negative:
If only the C band is present, the absence of any burgundy colour in A and B bands indicates that no Flu A/B antigen is detected in the specimen. The result is negative.
Positive:
FLU A/B positive: In addition to the presence of the C-line, If the test lines A and B appears at the same time, it means that there are both influenza A virus antigen and influenza B virus antigen in the sample, that is, the result is positive for FLU A and FLU B.
FLU A positive: In addition to the presence of the C-line, if the test line A appears, the test indicates the presence of FLU A antigen in the sample, that is, the result is positive for FLU A.
FLU B positive: In addition to the presence of the C-line, if the test line B appears, the test indicates the presence of FLU B antigen in the sample, that is, the result is positive for FLU B.
Invalid:
Control line fails to appear. Insufficient specimen volume or incorrect procedural techniques are the most likely reasons for control line failure. Review the procedure and repeat the test with a new test cassette. If the problem persists, discontinue using the test kit immediately and contact your local distributor.
Limitations
- Use fresh samples whenever possible.
- The COVID-19 and FLU A/B Antigen Test Kit will only indicate the presence of COVID-19/Influenza A/Influenza B Antigens in the specimen and should not be used as the sole basis for the diagnosis of COVID-19/Influenza A/Influenza B infections.
- Positive results do not rule out bacterial infection or co-infection with other viruses.
- A negative result for an individual subject indicates absence of detectable COVID-19 or Flu A/B antigen. However, a negative test result does not preclude the possibility of exposure to or infection with COVID-19 and Flu A/B.
- A negative result can occur if the quantity of the COVID-19 or Flu A/B antigen present in the specimen is below the detection limits of the assay, or failed to collect the COVID-19 or Flu A/B antigen in the nasal cavity of the patient.
- As with all diagnostic tests, a definitive clinical diagnosis should not be based on the result of a single test, but should only be made by the physician combined with clinical observations, patient history, recent exposures and epidemiological information.
- The accuracy of the test depends on the quality of the swab sample. False negatives may result from improper sample collection or storage.
Warnings and precautions
- Allow test cassette, antigen extraction buffer, specimen and/or controls to equilibrate to room temperature (15-30°C) prior to testing.
- For Professional In Vitro Diagnostic use only. Do not use after expiration date.
- These instructions must be strictly followed by a trained healthcare professional to achieve accurate results. All healthcare professionals have to read the instruction prior to performing a test.
- Do not use it if the tube/pouch is damaged or broken.
- Decontaminate and dispose of all specimens, reaction kits and potentially contaminated materials (i.e., swab, extraction tube, test device) in a biohazard container as if they were infectious waste and dispose according to applicable local regulations.
- The provided swabs in the package should be used only for nasopharyngeal specimen collection.
- Test is for single use only. Do not re-use under any circumstances.
- Do not perform the test in a room with strong air flow, i.e., electric fan or strong air-conditioning.
Since its establishment in August 2020, the joint UK Health Security Agency (UKHSA) Porton Down and University of Oxford SARS-CoV-2 lateral flow antigen test validation cell has evaluated over 160 lateral flow devices that have been referred by the Department of Health and Social Care.
Read further information about Panodyne COVID-19 ANTIGEN RAPID TEST KIT passing phase 3 validation: